
State description in quantum mechanics
 Bohr energy levels Bohr energy levels
|
Second Schrödinger equation 
|
[3. Schrödinger equations.]
State description in quantum mechanics.
The state S of a quantum system is completely described by a complex function
Ψ (q) depending from position q.
Let u be a classical physical quantity and let uop be the
corresponding quantum operator. The first Schrödinger equation is
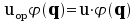 .
The eigenvalues ui are the admissible values of u.
.
The eigenvalues ui are the admissible values of u.
The complex function Ψ (q) can be represented as a sum of the
eigenfunctions:
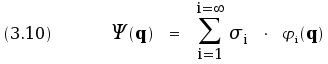
Complex numbers σi are called the expansion coefficients;
the square of their module determines the probability
PS( ui ) that u has the value
ui in the state S :
PS( ui ) = | σi |² .
The square of the module of Ψ (q) gives the probability that, in the
state S, the system is to be found in position q :
PS( q ) = | Ψ (q) |² .
The probability of every physical quantity, in the state S, is determined by the complex function
Ψ (q) together with the first Schrödinger equation, by means of the
following two steps:
- The eigenvalues of the first Schrödinger equation give the admissible values for that physical quantity.
- The representation of Ψ (q) as a sum of the eigenfunctions gives, by means of the expansion coefficients, the probability of every value.
 Bohr energy levels Bohr energy levels
|
Second Schrödinger equation 
|